Nogra Pharma is designing and developing SPPARMs that specifically target Immuno-Inflammatory pathways.

Selective PPARy Modulation
PPARs Modulation emerged as a model to explain the different pharmacological effects and gene modulating expression profiles induced by different PPAR ligands, and has been now validated with different compounds.
This model proposed that different PPAR ligands bind to the LBD of the receptor and, depending on their chemical structure, induce distinct conformational changes in the receptor, resulting in differential interaction with cofactors (coactivators and corepressors) and leading to differences in transcriptional expression of target genes (https://doi.org/10.1021/jm101360s ).
Modulators
Selective PPARγ Modulators (SPPARMs)
PPARγ is a ligand-inducible transcription receptor, that has a key role in organ homeostasis and downregulates inflammation and fibrosis. Thus PPARγ is a potential therapeutic target in a range of inflammatory and fibrotic diseases.
Different Ligands interact in distinct manner with the ligand-binding domain of PPARγ and determine the resultant transcriptional signature and, consequently, the pharmacological and safety profile of the ligand. (https://doi.org/10.1021/jm101360s)
Computational-chemistry-based studies allow the development of molecules that differentially activate PPARγ without inducing adverse effects.
Modulation
Selective PPAR Modulation (SPPARM)
SPPARMs: PPAR binders, structurally different from the known PPAR agonists, which compared to a full agonist modulate the expression of a limited number of genes. The pharmacological definition of SPPARM is a ligand that, compared to a full agonist, differentially induces specific receptor effects and in which dose-response relationships for various activities are uncoupled from each other. PPARγ conformation determines activity profile and is ligand dependent. PPARγ ligands include a wide variety of chemical structures. Ligands dock in various modes in the LBD pocket.
Agonists
SPPARMs vs PPARy agonists
The nonclinical and clinical safety data collected so far on GED-0507 and NAC-GED-0507 provide evidence to differentiate them vs PPARγ agonists.
Rational Drug Design of
New Chemical Entities, SPPARMs.
Based on computational chemistry and extensive SAR studies, Nogra Pharma developed a unique approach to design and select SPPARMs, encompassing the following steps:
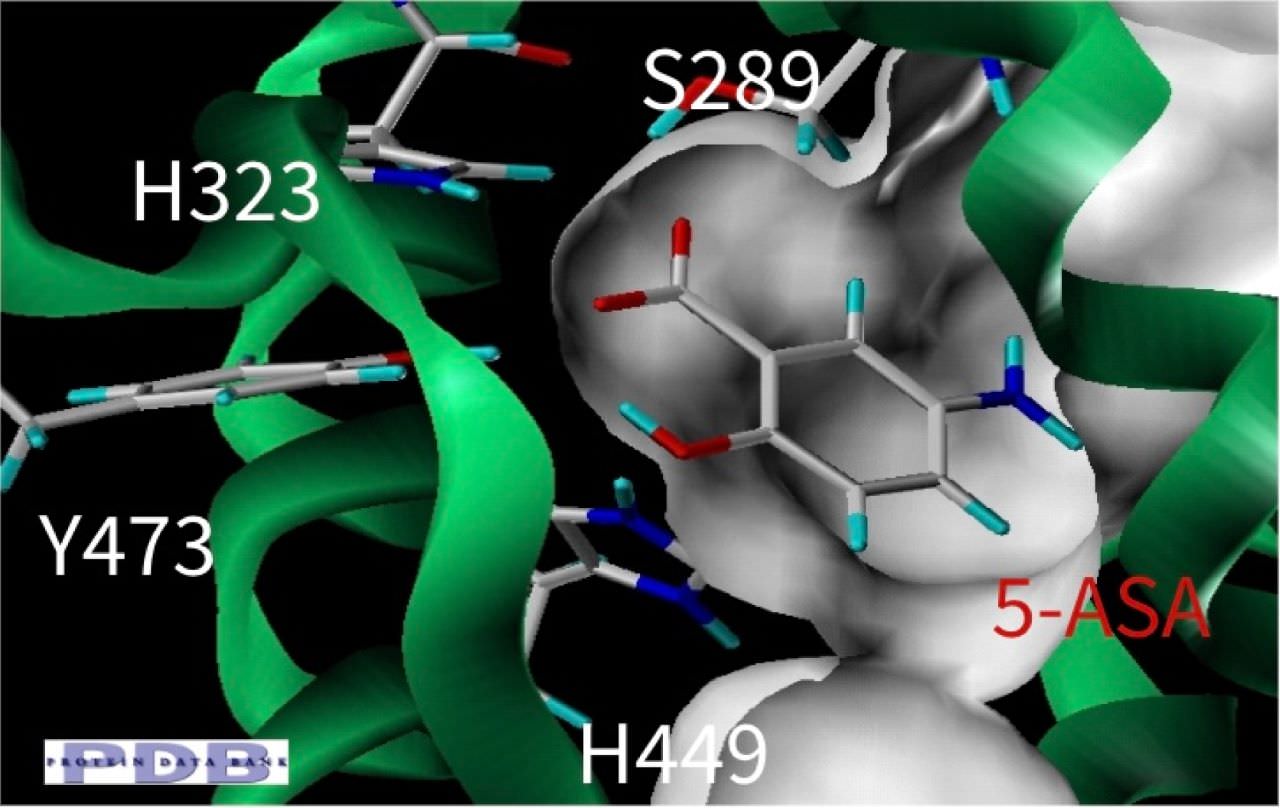
- Development of an in silico docking model based on the X-Ray crystal structure of PPARγ LBD complexed with binders.
- Rational design of compounds able to bind to PPARγ LBD
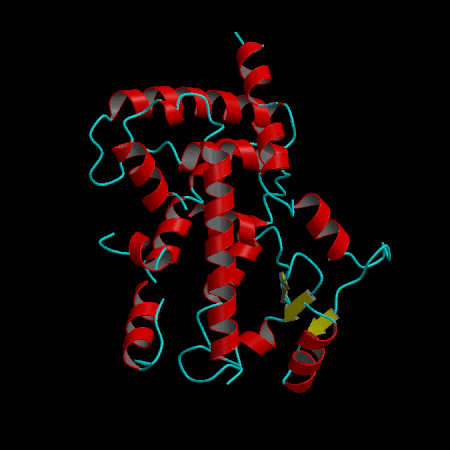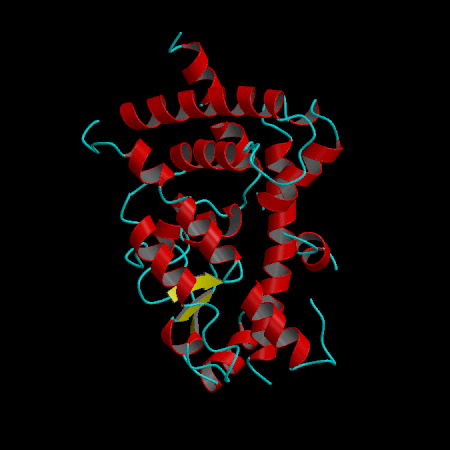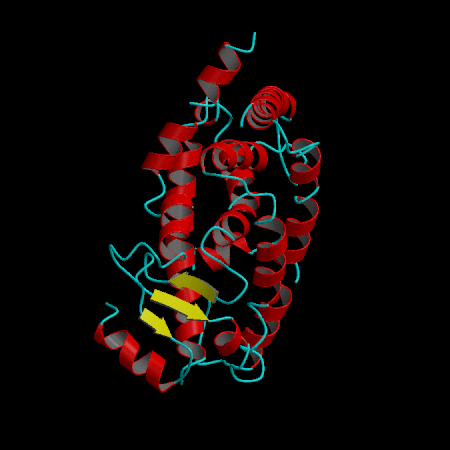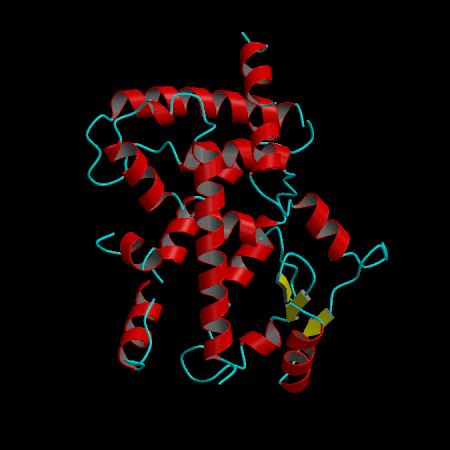
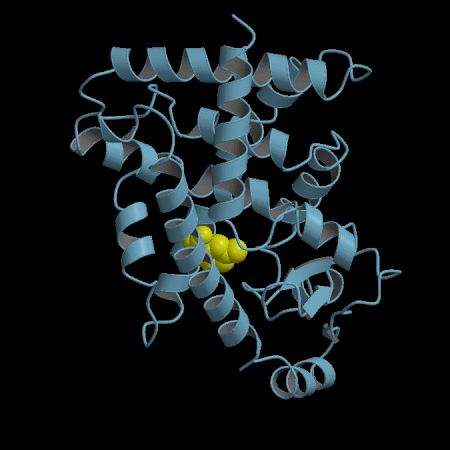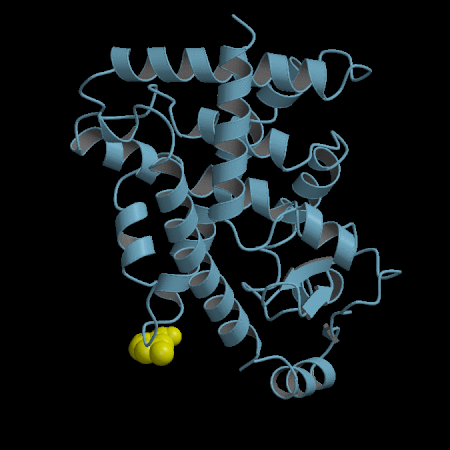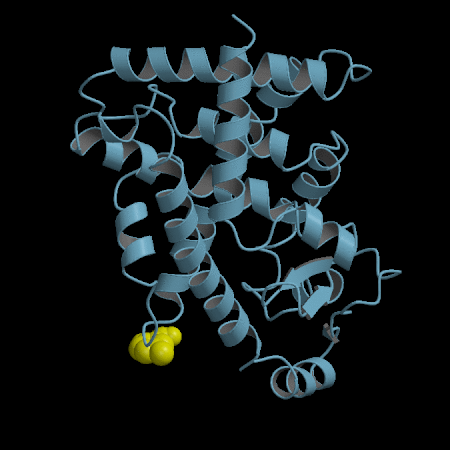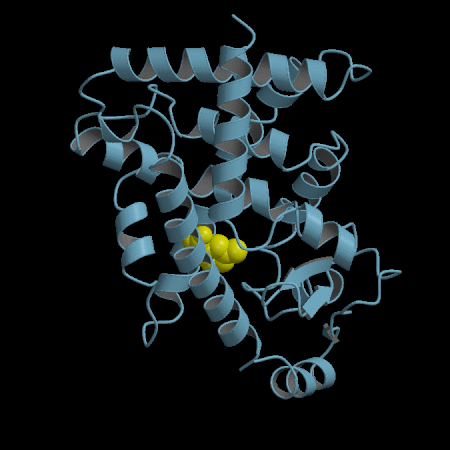
- Selection of compounds based on chemical accessibility, patentability, in silico predicted ADMET properties.
- Small scale synthesis and in vitro screening of the selected New Chemical Entities based on their safety and pharmacology profile.
Projects
Nogra Pharma possesses unique knowledge on how to design new SPPARMs that specifically target Immuno-Inflammatory pathways.
Target ID
NCEs design, Lead ID & Validation
Nonclinical Pharmacology & Toxicology
Formulation Development
CMC - DS & DP
Phase I Clinical Trial
Phase II Clinical Trial
PIVOTAL Clinical Trials
SPPARMs Platform
NAC-GED-0507 GEL
Acne Vulgaris
GED-0507 Tablet
Idiopathic Pulmonary Fibrosis (IPF)
New SPPARMS
IMID Immune-mediated inflammatory diseases

NAC-GED-0507
Topical Treatment of Acne
NAC-GED-0507 is an innovative New Chemical Entity for the treatment of Acne and Psoriasis.
Five completed clinical trials, more than 500 patients treated with active, very favorable safety profile.

GED-0507 Oral treatment
of Idiopathic Pulmonary Fibrosis (IPF)
GED-0507 attenuates lung fibrosis by counteracting myofibroblast transdifferentiation in vivo and in vitro. The project has completed the nonclinical development stage and is ready to enter the clinical development stage.

New SPPARMs (NCEs)
Ongoing in vitro screening
Undisclosed Indications

